Why is GMP essential? Weak quality medicines are not just a health and fitness hazard, but a squander of money for the two governments and unique buyers.
tasks from the impartial high quality device(s) really should not be delegated. These responsibilities must be described in producing and may contain, but not essentially be restricted to:
(one) Each and every producer and packer who packages an OTC drug solution (other than a dermatological, dentifrice, insulin, or lozenge product or service) for retail sale shall deal the solution in a tamper-evident offer, if this products is obtainable to the public although held available for purchase. A tamper-obvious package deal is one particular getting one or more indicators or barriers to entry which, if breached or missing, can moderately be anticipated to offer noticeable proof to consumers that tampering has happened. To lessen the likelihood of effective tampering also to boost the likelihood that consumers will learn if a product has been tampered with, the bundle is required for being distinct by layout or by using a number of indicators or boundaries to entry that employ an figuring out characteristic (e.
Grievances about marketed solutions have to be examined, the will cause of quality defects have to be investigated, and correct steps should be taken with respect into the faulty merchandise and to circumvent recurrence.
After the inspection closing Conference, you will receive a post inspection letter confirming any deficiencies discovered.
(b) The current good manufacturing practice polices Within this chapter as they pertain to drug items; in parts 600 by 680 of the chapter, since they pertain to medications which can be also Organic goods for human use; and partially 1271 of the chapter, as These are applicable to medication that happen to be also human cells, tissues, and cellular and tissue-centered products and solutions (HCT/Ps) and which might be medicine (subject matter to evaluate beneath an software submitted under part 505 in the act or under a Organic item license application under portion 351 of the general public Health Company Act); nutritional supplement and do not supersede the polices With this part Except the restrictions explicitly present if not.
(i) A few months once the expiration day of the final large amount of the drug merchandise that contains the Lively component If your expiration relationship duration of the drug merchandise is thirty days or fewer; or
Deciding upon an merchandise from entire text search engine results will carry you to definitely These results. Pressing enter during the lookup box will likely convey you to search engine results. History and even more information can be found in the Search & Navigation information.
A written history of important machines cleaning, upkeep click here (other than routine routine maintenance which include lubrication and adjustments), and use shall be A part of unique devices logs that show the day, time, item, and good deal variety of Each individual batch processed. If equipment is dedicated to manufacture of 1 item, then personal products logs are not necessary, presented that a lot or batches of this sort of product follow in numerical purchase and so are made in numerical sequence.
(a) An correctly discovered reserve sample which is agent of each and every whole lot in Each and every cargo of each and every active ingredient shall be retained. The reserve sample consists of no less than 2 times the quantity necessary for all tests demanded to ascertain whether or not the Lively component fulfills its recognized specs, apart from sterility and pyrogen screening. The retention time is as follows:
Proper SCALE: Catalent has the capability and integrated products and services to assistance any scale of application, from smaller click here orphan enhancement systems to huge-scale professional manufacturing. We offer a spread of apparatus scales to fulfill your demand from customers, at each stage from the product lifecycle.
(d) Acceptance requirements for your sampling and screening conducted by the quality Manage device shall be adequate to guarantee that batches of drug products meet up with Every single proper specification and suitable statistical top quality Manage criteria as being a situation for his or her approval and launch.
If you would like to comment on the current information, remember to utilize the 'Written content Comments' button down below for Directions on calling the issuing agency
Don’t contain particular or economic information and facts like your National Insurance variety or charge card particulars.
 Jonathan Taylor Thomas Then & Now!
Jonathan Taylor Thomas Then & Now!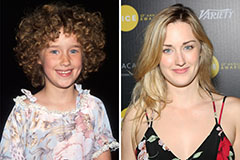 Ashley Johnson Then & Now!
Ashley Johnson Then & Now! Christina Ricci Then & Now!
Christina Ricci Then & Now! Seth Green Then & Now!
Seth Green Then & Now! Naomi Grossman Then & Now!
Naomi Grossman Then & Now!